views
Market Overview:
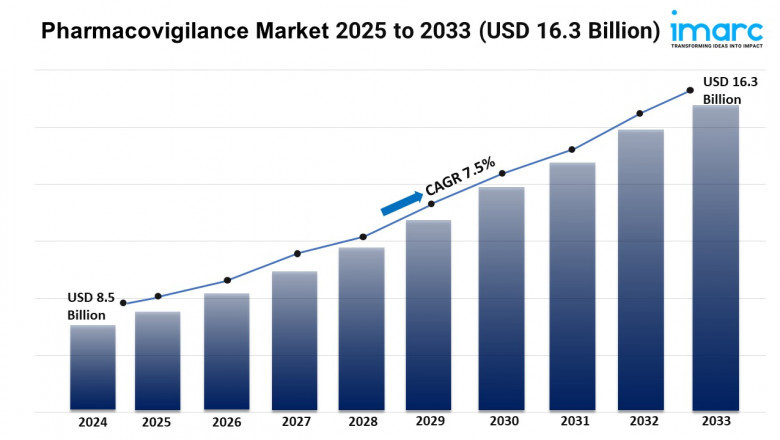
The pharmacovigilance market is experiencing rapid growth, driven by rising drug safety concerns, outsourcing to specialized providers, and ai and automation adoption. According to IMARC Group's latest research publication, "Pharmacovigilance Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2025-2033", offers a comprehensive analysis of the industry, which comprises insights on the global pharmacovigilance market share. The global market size was valued at USD 8.5 Billion in 2024. Looking forward, IMARC Group estimates the market to reach USD 16.3 Billion by 2033, exhibiting a CAGR of 7.5% from 2025-2033.

This detailed analysis primarily encompasses industry size, business trends, market share, key growth factors, and regional forecasts. The report offers a comprehensive overview and integrates research findings, market assessments, and data from different sources. It also includes pivotal market dynamics like drivers and challenges, while also highlighting growth opportunities, financial insights, technological improvements, emerging trends, and innovations. Besides this, the report provides regional market evaluation, along with a competitive landscape analysis.
Grab a sample PDF of this report: https://www.imarcgroup.com/pharmacovigilance-market/requestsample
Our report includes:
- Market Dynamics
- Market Trends And Market Outlook
- Competitive Analysis
- Industry Segmentation
- Strategic Recommendations
Factors Affecting the Growth of the Pharmacovigilance Industry:
- Rising Drug Safety Concerns:
The Pharmacovigilance market is experiencing significant growth due to increasing drug safety concerns worldwide. With the rapid development of novel therapies, including biologics and biosimilars, regulatory bodies and pharmaceutical companies are prioritizing adverse event monitoring. High-profile drug recalls and litigation cases have further emphasized the need for robust pharmacovigilance systems. Companies are investing in advanced analytics and AI-driven tools to detect risks early, ensuring compliance with stringent regulations like FDA’s FAERS and EU’s EudraVigilance. This trend is driving demand for real-time safety monitoring solutions, creating opportunities for innovation in the sector.
- Outsourcing to Specialized Providers:
Pharmaceutical firms are increasingly outsourcing pharmacovigilance activities to specialized CROs (Contract Research Organizations) to reduce costs and enhance efficiency. As drug pipelines expand, in-house teams struggle to manage large volumes of safety data, leading to partnerships with third-party experts. These providers offer scalable solutions, from case processing to risk management, leveraging global expertise and advanced technologies. The shift toward outsourcing is particularly prominent in emerging markets, where local regulatory knowledge is critical. This dynamic is reshaping the competitive landscape, with CROs expanding their service portfolios to meet growing client demands.
- AI and Automation Adoption:
Artificial Intelligence (AI) and automation are revolutionizing pharmacovigilance by streamlining adverse event reporting and signal detection. Traditional manual processes are being replaced by machine learning algorithms that analyze vast datasets quickly and accurately. Natural Language Processing (NLP) helps extract insights from unstructured sources like social media and electronic health records, improving early risk identification. Companies adopting these technologies gain a competitive edge through faster decision-making and reduced operational costs. As regulatory agencies encourage digital transformation, AI-driven pharmacovigilance is becoming a key differentiator in the market.
Leading Companies Operating in the Global Pharmacovigilance Industry:

- Accenture plc
- ArisGlobal LLC
- BioClinica Inc. (Cinven Partners LLP)
- Capgemini
- Cognizant
- International Business Machines Corporation
- ICON plc.
- IQVIA Inc.
- ITClinical
- Parexel International Corporation and Wipro Limited.
Pharmacovigilance Market Report Segmentation:
By Service Provider:

- In-house
- Contract Outsourcing
Contract outsourcing represents the largest segment due to the reliance of pharmaceutical companies on specialized service providers to manage pharmacovigilance activities, allowing them to focus on core operations while leveraging external expertise and cost efficiencies.
By Product Life Cycle:
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
Phase IV accounts for the majority of the market share as post-market surveillance becomes increasingly critical for monitoring drug safety and effectiveness in real-world settings, ensuring compliance with regulatory requirements and addressing emerging safety concerns post-approval.
By Type:
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
Spontaneous reporting exhibits a clear dominance in the market owing to its rising utilization in collecting adverse event data, leveraging healthcare professionals, patients, and other stakeholders to report adverse reactions voluntarily.
By Process Flow:
- Case Data Management
- Case Logging
- Case Data Analysis
- Medical Reviewing and Reporting
- Signal Detection
- Adverse Event Logging
- Adverse Event Analysis
- Adverse Event Review and Reporting
- Risk Management System
- Risk Evaluation System
- Risk Mitigation System
Signal detection holds the biggest market share driven by its pivotal role in identifying potential safety concerns by analyzing pharmacovigilance data to detect statistical associations between drugs and adverse events, enabling proactive risk management and regulatory compliance.
By Therapeutic Area:
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
Oncology dominates the market, with escalating demand for robust pharmacovigilance strategies to monitor the safety profiles of anticancer drugs and manage unique safety challenges associated with oncology treatments.
By End Use:
- Pharmaceuticals Companies
- Biotechnology Companies
- Medical Device Companies
- Others
Pharmaceutical companies represent the largest segment, which can be attributed to their crucial role in developing, manufacturing, and marketing drugs, necessitating comprehensive pharmacovigilance programs to ensure drug safety and regulatory compliance throughout the product lifecycle.
Regional Insights:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
North America enjoys the leading position in the pharmacovigilance market on account of its well-established regulatory framework, advanced healthcare infrastructure, high pharmaceutical expenditure, and presence of major pharmaceutical companies.
Research Methodology:
The report employs a comprehensive research methodology, combining primary and secondary data sources to validate findings. It includes market assessments, surveys, expert opinions, and data triangulation techniques to ensure accuracy and reliability.
Note: If you require specific details, data, or insights that are not currently included in the scope of this report, we are happy to accommodate your request. As part of our customization service, we will gather and provide the additional information you need, tailored to your specific requirements. Please let us know your exact needs, and we will ensure the report is updated accordingly to meet your expectations.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145













Comments
0 comment