views
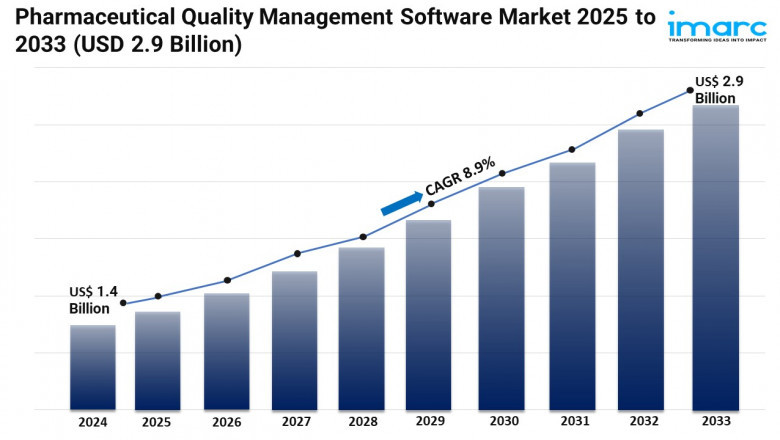
IMARC Group’s report titled “Pharmaceutical Quality Management Software Market Report by Solution Type (Corrective Action Preventive Action (CAPA) Management, Audit Management, Document Management, Change Management, Training Management, Risk Management, Complaints Management, Regulatory and Compliance Management, Non-Conformances Handling, Supplier Quality Management, Inspection Management, Equipment Management, and Others), Deployment Model (On-Cloud, Web-Based, On-Premises), Enterprise Size (Small and Medium-sized Enterprises, Large Enterprises), End User (Pharmaceutical, Life Sciences, and Biotechnology Companies, Contract Research Organizations, and Others), and Region 2025-2033”, Offers a comprehensive analysis of the industry, which comprises insights on the global pharmaceutical quality management software market share. The global market size reached USD 1.4 Billion in 2024. Looking forward, IMARC Group expects the market to reach USD 2.9 Billion by 2033, exhibiting a growth rate (CAGR) of 8.9% during 2025-2033.
Factors Affecting the Growth of the Pharmaceutical Quality Management Software Industry:
- Increasing Regulatory Compliance Requirements:
Regulatory agencies such as FDA along with EMA and WHO now scrutinize the pharmaceutical industry intensively which requires pharmaceutical organizations to invest in advanced quality management software (QMS). Health authorities enforce rigorous regulations which demand detailed documentation together with system-based traceability methods and instantaneous quality metric monitoring. Leading pharmaceutical companies purchase QMS solutions to streamline compliance initiatives and minimize risks that harm compliance status and boost operational productivity. The expanding regulatory environment drives QMS software adoption which will create new opportunities for innovation in compliance management system development.
- Emphasis on Risk Management & Quality Improvement:
Pharmaceutical companies dedicate efforts to active risk management while maintaining continuous improvements to quality outcomes because of rising development and manufacturing complexity. Through real-time risk assessments QMS software lets organizations perform Six Sigma and Lean methodology implementation to maximize process performance and minimize operational waste. Advanced analytic functions together with reporting engines enable organizations to find quality patterns while improving their decisions and operating with more effectiveness. Organizations embrace proactive quality management systems because of increasing need for advanced QMS solutions.
- Digital Transformation & Industry 4.0 Integration:
The pharmaceutical industry has adopted digital transformation through artificial intelligence and internet of things and machine learning technologies which substantially modify their quality management systems. The modern QMS implements different technologies through its software which delivers predictive analytics and automatic workflow management and provides real-time manufacturing environment oversight. IoT devices deliver essential quality information to clients while AI-driven analytics detect potential problems before edition to ensure better accuracy in results. Modern QMS solution adoption will increase due to ongoing digital transformation speed.
Grab a sample PDF of this report: https://www.imarcgroup.com/pharmaceutical-quality-management-software-market/requestsample
Leading Companies Operating in the Pharmaceutical Quality Management Software Industry:

- AssurX Inc.
- ComplianceQuest
- Dassault Systèmes SE (Dassault Group)
- ETQ LLC (Hexagon AB)
- Ideagen
- IQVIA Inc.
- Mastercontrol Inc.
- Qualio
- Veeva Systems Inc
Pharmaceutical Quality Management Software Market Report Segmentation:
Breakup By Solution Type:
- Corrective Action Preventive Action (CAPA) Management
- Audit Management
- Document Management
- Change Management
- Training Management
- Risk Management
- Complaints Management
- Regulatory and Compliance Management
- Non-Conformances Handling
- Supplier Quality Management
- Inspection Management
- Equipment Management
- Others
Breakup By Deployment Model:

- On-Cloud
- Web-Based
- On-Premises
Breakup By Enterprise Size:
- Small and Medium-sized Enterprises
- Large Enterprises
Breakup By End User:
- Pharmaceutical, Life Sciences, and Biotechnology Companies
- Contract Research Organizations
- Others
Regional Insights:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
Pharmaceutical Quality Management Software Market Trends:
The pharmaceutical QMS market is witnessing a surge in cloud-based solutions, offering scalability, flexibility, and remote accessibility. User-friendly interfaces and mobile compatibility are gaining traction, enabling seamless quality management. Additionally, the integration of advanced analytics within QMS software is empowering organizations with data-driven insights for continuous improvement. As regulatory landscapes and industry demands evolve, the adoption of agile, modern QMS solutions is becoming crucial for pharmaceutical companies seeking to maintain compliance and optimize quality management.
Note: If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1–631–791–1145













Comments
0 comment