views
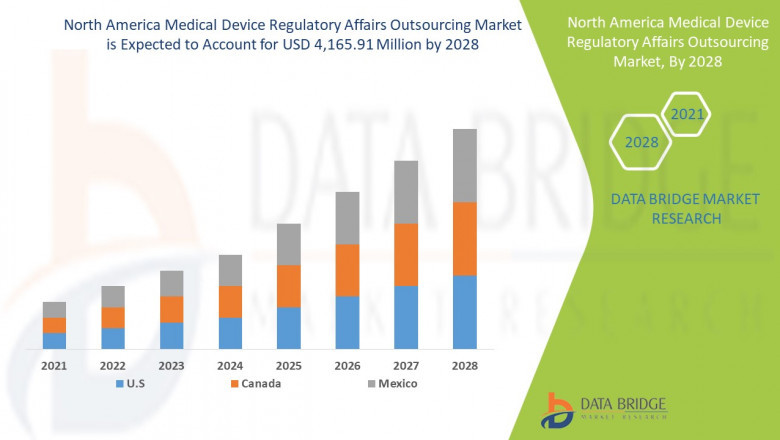
The North America Medical Device Regulatory Affairs Outsourcing Market sector is undergoing rapid transformation, with significant growth and innovations expected by 2028. In-depth market research offers a thorough analysis of market size, share, and emerging trends, providing essential insights into its expansion potential. The report explores market segmentation and definitions, emphasizing key components and growth drivers. Through the use of SWOT and PESTEL analyses, it evaluates the sector’s strengths, weaknesses, opportunities, and threats, while considering political, economic, social, technological, environmental, and legal influences. Expert evaluations of competitor strategies and recent developments shed light on geographical trends and forecast the market’s future direction, creating a solid framework for strategic planning and investment decisions.
Get a Sample PDF of Report - https://www.databridgemarketresearch.com/request-a-sample/?dbmr=north-america-medical-device-regulatory-affairs-outsourcing-market
Report Scope and Market Segmentation
Which are the driving factors of the North America Medical Device Regulatory Affairs Outsourcing Market?
The driving factors of the North America Medical Device Regulatory Affairs Outsourcing Market are multifaceted and crucial for its growth and development. Technological advancements play a significant role by enhancing product efficiency, reducing costs, and introducing innovative features that cater to evolving consumer demands. Rising consumer interest and demand for keyword-related products and services further fuel market expansion. Favorable economic conditions, including increased disposable incomes, enable higher consumer spending, which benefits the market. Supportive regulatory environments, with policies that provide incentives and subsidies, also encourage growth, while globalization opens new opportunities by expanding market reach and international trade.
North America Medical Device Regulatory Affairs Outsourcing Market - Competitive and Segmentation Analysis:
**Segments**
The North America Medical Device Regulatory Affairs Outsourcing Market is segmented based on service type, application, and end-user. By service type, the market is divided into regulatory writing and publishing, clinical trial applications, regulatory submissions, product registration and other services. The regulatory writing and publishing segment is expected to witness significant growth due to the increasing demand for comprehensive regulatory documentation and the need for accurate and timely submissions to regulatory authorities. In terms of application, the market is categorized into In-vitro diagnostic devices, diagnostic imaging devices, dental devices, orthopedic devices, cardiovascular devices, endoscopic devices, and others. The orthopedic devices segment is anticipated to experience substantial growth attributed to the rising prevalence of musculoskeletal disorders and the increasing geriatric population. Furthermore, based on end-user, the market is segmented into medical device companies, contract research organizations (CROs), and others. The medical device companies segment is projected to dominate the market owing to the growing complexity of regulatory requirements and the need for specialized expertise in navigating the regulatory landscape.
**Market Players**
- ICON plc
- Parexel International Corporation
- PRA Health Sciences
- Covance Inc.
- IQVIA
- Freyr Solutions
- Weinberg Group Inc.
- WDPrx - Woodfield Pharmaceutical, LLC
- Criterium Inc.
- MakroCare
The North America Medical Device Regulatory Affairs Outsourcing Market is witnessing robust growth driven by several key factors. One of the primary growth drivers is the increasing complexity of regulatory requirements for medical devices. With stringent regulations and evolving compliance standards, medical device companies are increasingly turning to outsourcing partners with specialized regulatory expertise to navigate the regulatory landscape efficiently. Additionally, the rising demand for innovative medical devices, coupled with the need for expedited market approvals, is fueling the demand for regulatory affairs outsourcing services. Outsourcing regulatory affairs activities allows companies to focus on core competencies such as research and development while ensuring compliance with regulatory requirements.
Furthermore, the growing trend of strategic partnerships and collaborations between medical device companies and outsourcing service providers is contributing to market growth. These partnerships enable companies to leverage the expertise of outsourcing providers in navigating complex regulatory processes, accelerating time-to-market, and enhancing operational efficiency. Moreover, the increasing adoption of advanced technologies such as artificial intelligence (AI) and machine learning in regulatory affairs outsourcing is expected to drive market growth. AI-powered solutions offer capabilities such as automated document management, regulatory intelligence, and compliance monitoring, thereby streamlining regulatory processes and enhancing decision-making.
Despite the positive growth prospects, the North America Medical Device Regulatory Affairs Outsourcing Market faces certain challenges. One of the key challenges is the lack of harmonization in regulatory standards across different geographies. Variations in regulatory requirements among different countries necessitate specialized knowledge and expertise to ensure compliance, posing a challenge for companies operating in multiple markets. Additionally, data privacy and security concerns present a significant challenge for outsourcing regulatory affairs activities, especially in light of increasing cybersecurity threats and regulations such as GDPR.
In conclusion, the North America Medical Device Regulatory Affairs Outsourcing Market is poised for significant growth driven by the increasing complexity of regulatory requirements, the demand for innovative medical devices, and the trend of strategic partnerships. By leveraging specialized regulatory expertise, advanced technologies, and collaborative partnerships, market players can capitalize on the growing opportunities in the regulatory affairs outsourcing sector.
https://www.databridgemarketresearch.com/reports/north-america-medical-device-regulatory-affairs-outsourcing-market
Explore Further Details about This Research North America Medical Device Regulatory Affairs Outsourcing Market Report https://www.databridgemarketresearch.com/reports/north-america-medical-device-regulatory-affairs-outsourcing-market
Key Benefits for Industry Participants and Stakeholders: –
- Industry drivers, trends, restraints, and opportunities are covered in the study.
- Neutral perspective on the North America Medical Device Regulatory Affairs Outsourcing Market scenario
- Recent industry growth and new developments
- Competitive landscape and strategies of key companies
- The Historical, current, and estimated North America Medical Device Regulatory Affairs Outsourcing Market size in terms of value and size
- In-depth, comprehensive analysis and forecasting of the North America Medical Device Regulatory Affairs Outsourcing Market
Key Questions Answered:
1. What is the North America Medical Device Regulatory Affairs Outsourcing Market?
2. How big is the North America Medical Device Regulatory Affairs Outsourcing Market?
3. What is the growth rate of the North America Medical Device Regulatory Affairs Outsourcing Market?
4. What are the key drivers of the North America Medical Device Regulatory Affairs Outsourcing Market?
5. Which region dominates the North America Medical Device Regulatory Affairs Outsourcing Market?
6. Who are the major players in the North America Medical Device Regulatory Affairs Outsourcing Market?
7. What segments are included in the North America Medical Device Regulatory Affairs Outsourcing Market?
8. What are the challenges facing the North America Medical Device Regulatory Affairs Outsourcing Market?
9. What is the future outlook for the North America Medical Device Regulatory Affairs Outsourcing Market?
10. How can companies benefit from the North America Medical Device Regulatory Affairs Outsourcing Market?
Browse More Reports:
North America Confectionery Processing Equipment Market – Industry Trends and Forecast
Scleroderma Therapeutics Market – Industry Trends and Forecast
Asia-Pacific Scleroderma Therapeutics Market – Industry Trends and Forecast
Europe Scleroderma Therapeutics Market - Industry Trends and Forecast
Middle East and Africa Scleroderma Therapeutics Market - Industry Trends and Forecast
North America Scleroderma Therapeutics Market - Industry Trends and Forecast
5G Enterprise Market – Industry Trends and Forecast
Drug Discovery Outsourcing Market – Industry Trends and Forecast
Beverage Packaging Market – Industry Trends and Forecast
Solid State Relay Market - Industry Trends and Forecasts to 2028
In Vitro Toxicity Testing in Chemical Market – Industry Trends and Forecast
Breakthrough Therapy (BT) Designation Market – Industry Trends and Forecast
Asia-Pacific Collaborative Robot Market – Industry Trends and Forecast
Europe Collaborative Robot Market - Industry Trends and Forecast
Middle East and Africa Collaborative Robot Market - Industry Trends and Forecast
Data Bridge Market Research:
Today's trends are a great way to predict future events!
Data Bridge Market Research is a market research and consulting company that stands out for its innovative and distinctive approach, as well as its unmatched resilience and integrated methods. We are dedicated to identifying the best market opportunities, and providing insightful information that will help your business thrive in the marketplace. Data Bridge offers tailored solutions to complex business challenges. This facilitates a smooth decision-making process. Data Bridge was founded in Pune in 2015. It is the product of deep wisdom and experience.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC: +653 1251 978













Comments
0 comment