views
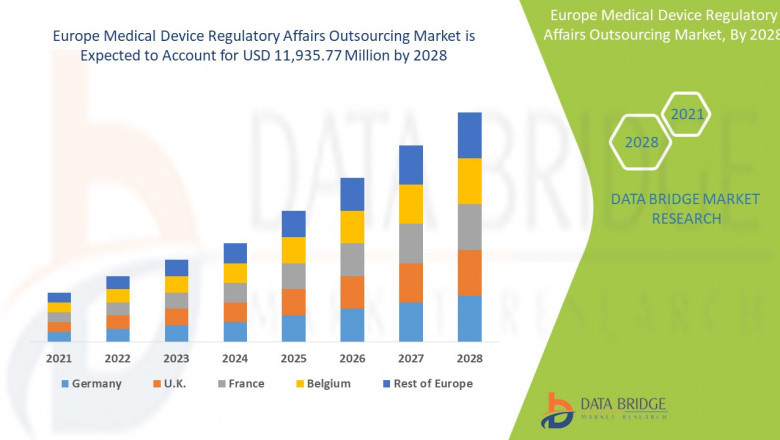
The Europe Medical Device Regulatory Affairs Outsourcing Market sector is undergoing rapid transformation, with significant growth and innovations expected by 2028. In-depth market research offers a thorough analysis of market size, share, and emerging trends, providing essential insights into its expansion potential. The report explores market segmentation and definitions, emphasizing key components and growth drivers. Through the use of SWOT and PESTEL analyses, it evaluates the sector’s strengths, weaknesses, opportunities, and threats, while considering political, economic, social, technological, environmental, and legal influences. Expert evaluations of competitor strategies and recent developments shed light on geographical trends and forecast the market’s future direction, creating a solid framework for strategic planning and investment decisions.
Get a Sample PDF of Report - https://www.databridgemarketresearch.com/request-a-sample/?dbmr=europe-medical-device-regulatory-affairs-outsourcing-market
Report Scope and Market Segmentation
Which are the driving factors of the Europe Medical Device Regulatory Affairs Outsourcing Market?
The driving factors of the Europe Medical Device Regulatory Affairs Outsourcing Market are multifaceted and crucial for its growth and development. Technological advancements play a significant role by enhancing product efficiency, reducing costs, and introducing innovative features that cater to evolving consumer demands. Rising consumer interest and demand for keyword-related products and services further fuel market expansion. Favorable economic conditions, including increased disposable incomes, enable higher consumer spending, which benefits the market. Supportive regulatory environments, with policies that provide incentives and subsidies, also encourage growth, while globalization opens new opportunities by expanding market reach and international trade.
Europe Medical Device Regulatory Affairs Outsourcing Market - Competitive and Segmentation Analysis:
**Segments**
The Europe Medical Device Regulatory Affairs Outsourcing Market is segmented based on service, service type, and application. By service, the market is segmented into regulatory writing and publishing, regulatory submissions, clinical trial applications, and product registration. Regulatory writing and publishing involve the preparation of documents required for regulatory submissions, while regulatory submissions encompass the actual submission process to regulatory authorities. Clinical trial applications involve the preparation and submission of documents necessary for conducting clinical trials, and product registration includes the registration of medical devices with regulatory bodies. By service type, the market is segmented into in-house outsourcing and off-site outsourcing. In-house outsourcing refers to the outsourcing of regulatory affairs activities to a team within the company, while off-site outsourcing involves the engagement of external service providers for regulatory affairs support. By application, the market is segmented into medical devices, in-vitro diagnostic devices, and others.
**Market Players**
- ICON plc
- Freyr
- PAREXEL International Corporation
- Covance Inc.
- Criterium, Inc.
- Weinberg Medical Physics LLC
- Promedica International
- ProRelix Research
- MakroCare
- CROMSOURCE
The Europe Medical Device Regulatory Affairs Outsourcing Market is witnessing significant growth due to various factors. One of the key growth drivers is the increasing complexity of regulatory requirements in the medical device industry. Regulatory bodies in Europe are continuously updating and tightening regulations to ensure the safety and efficacy of medical devices, which has led to a greater demand for regulatory affairs outsourcing services. Additionally, the growing number of medical device companies in Europe, particularly small and medium-sized enterprises (SMEs), are seeking regulatory affairs support to navigate the complex regulatory landscape efficiently. Outsourcing regulatory affairs activities allows companies to access specialized expertise and resources, enabling them to streamline the regulatory approval process and accelerate time-to-market for their products.
Furthermore, the trend towards globalization in the medical device industry is driving the need for regulatory affairs outsourcing services. With companies expanding their operations to international markets, they must comply with diverse regulatory requirements across different regions. Outsourcing regulatory affairs helps companies ensure compliance with local regulations and standards, facilitating market access and expansion opportunities. Moreover, the increasing focus on innovation and technological advancements in medical devices is fueling the demand for regulatory affairs outsourcing. Companies are investing in R&D to develop cutting-edge medical technologies, and outsourcing regulatory affairs allows them to focus on innovation while ensuring regulatory compliance.
Despite the growth prospects, the Europe Medical Device Regulatory Affairs Outsourcing Market faces certain challenges. One of the primary challenges is the lack of harmonization in regulatory requirements across European countries. Each country has its own regulatory framework and submission requirements, resulting in complexity and inconsistency for companies operating in multiple markets. This fragmentation complicates the outsourcing process and adds to the regulatory burden for medical device companies. Additionally, the stringent quality and data privacy regulations in Europe pose challenges for regulatory affairs outsourcing providers in ensuring compliance and data security.
In conclusion, the Europe Medical Device Regulatory Affairs Outsourcing Market is poised for significant growth driven by the increasing regulatory complexity, globalization of the industry, and focus on innovation. Companies are turning to outsourcing services to navigate the evolving regulatory landscape efficiently and expedite market entry. However, challenges such as regulatory fragmentation and data privacy regulations need to be addressed to sustain the market growth. By leveraging the expertise of leading market players like ICON plc, Freyr, and PAREXEL International Corporation, companies can optimize their regulatory affairs processes and enhance their competitive position in the market.
https://www.databridgemarketresearch.com/reports/europe-medical-device-regulatory-affairs-outsourcing-market
Explore Further Details about This Research Europe Medical Device Regulatory Affairs Outsourcing Market Report https://www.databridgemarketresearch.com/reports/europe-medical-device-regulatory-affairs-outsourcing-market
Key Benefits for Industry Participants and Stakeholders: –
- Industry drivers, trends, restraints, and opportunities are covered in the study.
- Neutral perspective on the Europe Medical Device Regulatory Affairs Outsourcing Market scenario
- Recent industry growth and new developments
- Competitive landscape and strategies of key companies
- The Historical, current, and estimated Europe Medical Device Regulatory Affairs Outsourcing Market size in terms of value and size
- In-depth, comprehensive analysis and forecasting of the Europe Medical Device Regulatory Affairs Outsourcing Market
Key Questions Answered:
1. What is the Europe Medical Device Regulatory Affairs Outsourcing Market?
2. How big is the Europe Medical Device Regulatory Affairs Outsourcing Market?
3. What is the growth rate of the Europe Medical Device Regulatory Affairs Outsourcing Market?
4. What are the key drivers of the Europe Medical Device Regulatory Affairs Outsourcing Market?
5. Which region dominates the Europe Medical Device Regulatory Affairs Outsourcing Market?
6. Who are the major players in the Europe Medical Device Regulatory Affairs Outsourcing Market?
7. What segments are included in the Europe Medical Device Regulatory Affairs Outsourcing Market?
8. What are the challenges facing the Europe Medical Device Regulatory Affairs Outsourcing Market?
9. What is the future outlook for the Europe Medical Device Regulatory Affairs Outsourcing Market?
10. How can companies benefit from the Europe Medical Device Regulatory Affairs Outsourcing Market?
Browse More Reports:
Pulmonary Function Testing Market – Industry Trends and Forecast
Automotive Braking Systems Market – Industry Trends and Forecast
Baking Ingredients Market – Industry Trends and Forecast
Edible Oils Market – Industry Trends and Forecast
Deodorization Systems Market – Industry Trends and Forecast
Footwear Sole Material Market – Industry Trends and Forecast
Pipe and Tank Coatings Market – Industry Trends and Forecast
China Silicon Anode Material Battery Market – Industry Trends and Forecast
Japan Silicon Anode Material Battery Market – Industry Trends and Forecast
Korea Silicon Anode Material Battery Market – Industry Trends and Forecast
Fractional Horsepower (FHP) Motors Market – Industry Trends and Forecast
Ice Lollies Market – Industry Trends and Forecast
Asia-Pacific Confectionery Processing Equipment Market – Industry Trends and Forecast
Europe Confectionery Processing Equipment Market – Industry Trends and Forecast
Middle East and Africa Confectionery Processing Equipment Market – Industry Trends and Forecast
Data Bridge Market Research:
Today's trends are a great way to predict future events!
Data Bridge Market Research is a market research and consulting company that stands out for its innovative and distinctive approach, as well as its unmatched resilience and integrated methods. We are dedicated to identifying the best market opportunities, and providing insightful information that will help your business thrive in the marketplace. Data Bridge offers tailored solutions to complex business challenges. This facilitates a smooth decision-making process. Data Bridge was founded in Pune in 2015. It is the product of deep wisdom and experience.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC: +653 1251 978













Comments
0 comment